Lean Quality Management:
Resolving the conflict between Control and Innovation
As the old adage goes, “ a dog with two masters will starve “ and when any organisation, political party or even us as individuals are pulled in two opposing directions it will usually end in tears.
But that is essentially what is happening in medical device companies today.
Let me explain...
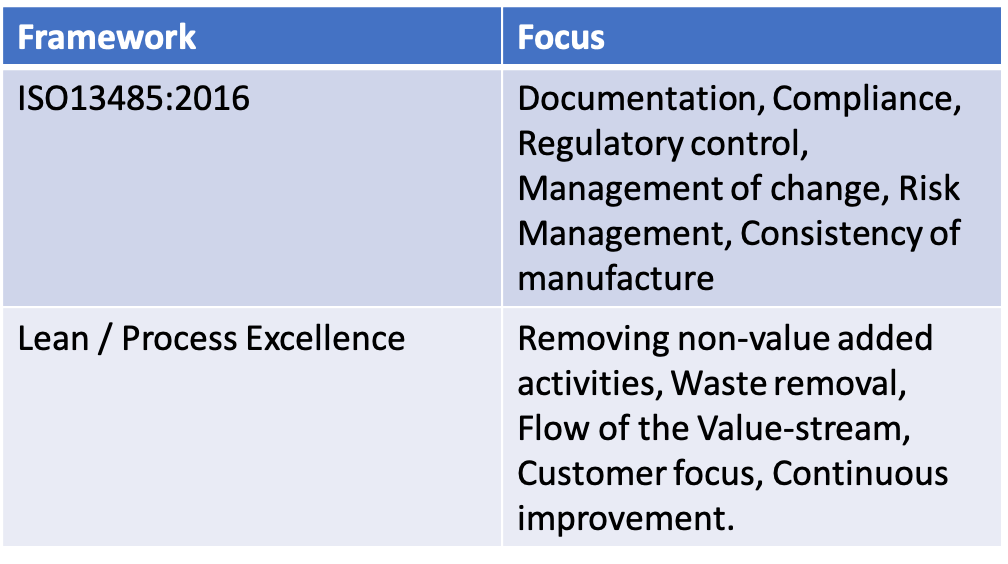
The Quality Management standard to ensure that medical devices are safe and effective is ISO13485. It differs in a number of respects to the ISO9001 series, with which it shares similarities. Whilst ISO9001 has a strong focus on improving customer satisfaction, ISO13485 is more concerned with ensuring the medical device does no harm and is effective. Whilst ISO9001 has strong emphasis on continuous improvement, ISO13485 is primarily focused on maintaining the effectiveness of the Quality Management System (QMS).
As a gross over-simplification, ISO9001 encourages change, whilst ISO13845 encourages consistency of the QMS and dependability of manufacture.
As such, the custodian of the QMS, usually the VP of Quality, is focused to ensure resources are aligned such that these regulatory requirements are met and control is maintained. Adhering to the requirements of ISO13485, isn’t a choice, it's a regulatory requirement.
But medical device companies are no different to other businesses. They need to make a profit, react swiftly to customer needs and continually look to innovate to ensure the business is efficient, effective and agile.
Over the last 30 years two methodologies have gained huge traction in making organisations more efficient and customer focused: lean and Process Excellence. Combined, they have absolute focus on reducing variation, removing waste and meeting customers' often changing needs. Innovation is encouraged to meets these needs. As such the custodian of these improvement methodologies, often the VP of Operations, ensues that there is laser focus on improvement.
This puts medical device companies with a potential conflict ie: the need to ensure control and reduce risk, or encourage innovation and take risks.
This creates specific challenges. Resources need to be aligned, effort not duplicated and methodologies not allowed to clash. There is huge potential for mixed messaging in organisations, as the two approaches are promoted. In truth a highly compliant organisation can be inefficient, unable to react to customer needs and will eventually perish. Conversely highly nimble innovative medical device companies face severe regulatory challenges if control of design and manufacture is not ensured.
In truth both needs, control and innovation, require satisfying. This is even more so with the Medical Device Regulations now putting specific expectation to:-
.. “ establish, document, implement, maintain, keep up to date and continually improve a quality management system.”
Given these two distinct and often competing needs, it is surprising how often the Quality and Operational functions do not speak the same language or understand the challenges each are facing ?
So how can this be resolved ?
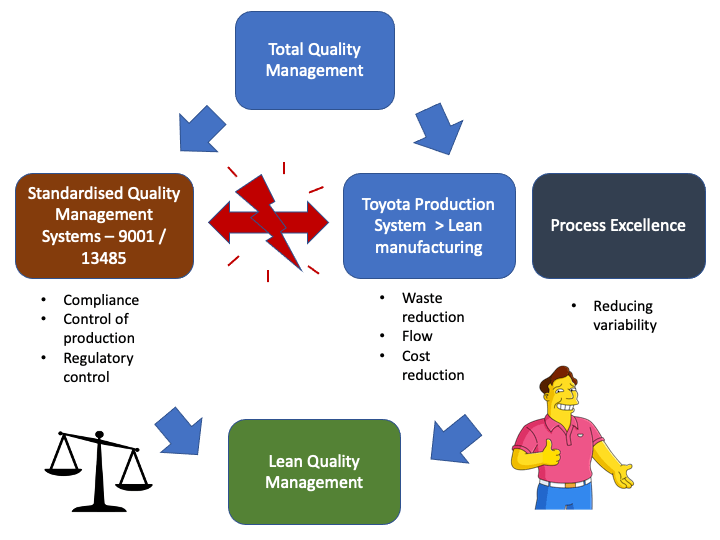
One approach is to leverage aspects of improvement methodologies, specifically the focus on understanding customer needs to ensure that the QMS is both compliant, in control and
also innovative - ie: Build a lean based
QMS.
Datod
has a number of training modules demonstrating how this can be achieved with practical examples how to build a lean based QMS with appropriate KPIs.
Either way unless medical device companies are able to satisfy the two masters of control and innovation they will potentially perish or be replaced by those that can.
Being able to satisfy both masters is a real win : win.