IVDR, Brexit and Covid: How your Quality Management System can help you weather the storm

Firstly, through the IVDR the industry is going though vastly increased regulatory scrutiny. Previously only 15% diagnostic tests required registration, now it is close to 85% . In addition, the requirements for demonstrating clinical performance are far higher now and apply to everything, even legacy products. The number of notifying bodies operating pre-IVDR has gone from around 170 to just a handful. Essentially the regulatory bar is far higher, the financial costs greater, the regulatory resources stretched and it can be argued that innovation potentially stifled, as start-ups are driven away by the extreme regulatory hurdles to get to market.
Secondly, Brexit. Like it or not, it has happened. The UK is no longer in the EU and this has brought very specific challenges for all UK companies wanting to trade with the EU, a particular issue for diagnostic companies with their diverse supply chains and often hard to source raw materials. Not to mention a potentially diverging set of regulatory requirements for companies wanting to trade within the UK.
Thirdly Covid. The pandemic has made any form of collaborative activity many-fold more difficult, be it hosting audits to demonstrate compliance, designing products, to connecting and supporting end-customers.
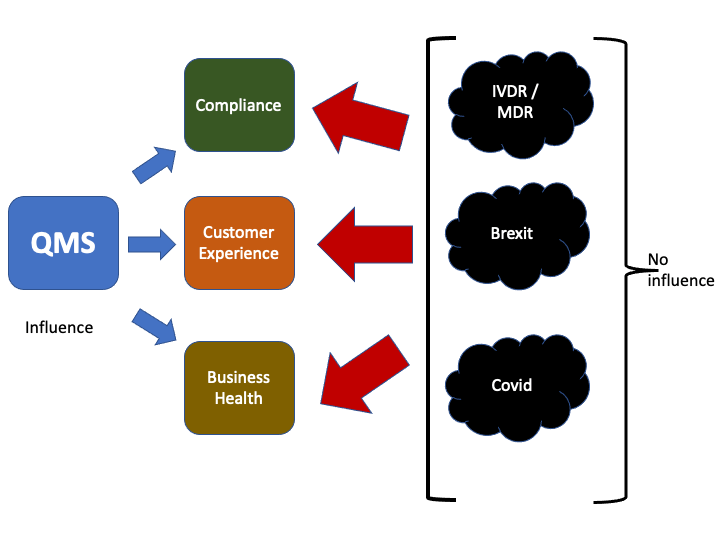
So, what can diagnostic companies do in the face of these challenges given the three stressors, IVDR/Brexit and Covid, they have little or no influence over ?
Well, the one aspect of the business diagnostics companies do have influence over is the QMS, and in such times a QMS that is cumbersome, inefficient and inflexible may be the final straw for many companies. Whilst, those that are ahead of the game and developing, nimble, stream-lined and efficient quality systems will not only weather the current storms but will be better placed to exploit the opportunities ahead.
If ever there was a time to invest in your QMS it is now, and it shouldn’t cost the earth.
[1] MedTech Europe 2018
[2] Oliver, P 2021. MediData. The Perfect Storm for Medical Device and In Vitro Diagnostics Companies in Europe. https://www.medidata.com/en/life-science-resources/medidata-blog/the-perfect-storm-for-medical-device-and-in-vitro-diagnostics-companies-in-europe