One of the successes in terms of road safety in the UK has been the year-on-year reduction in deaths due to drink-driving, accounting for over 1600 lost lives per year in the late 1970s reducing to just over 200 by 2016. Whilst this is partly due to advances in car safety, and the remaining deaths the result of a hard-core of drivers who continue to drive under the influence, over the years the number of lives tragically lost on roads through drink has been dramatically reduced.
However, since the late 1960s the drink drive legislation has more or less stayed the same.
So, if the law itself hasn’t made the difference, what has ?
Essentially attitudes to driving under the influence have dramatically changed over the years. It is no longer socially acceptable to get behind the wheel intoxicated. Over the decades there has been a seismic change in attitudes, and one that has been actively managed.
So, what has this got to do with the world of business, quality systems and healthcare ?
It demonstrates you can have all the procedures and rules in the world but it will not make a jot of difference if you don’t change behaviours.
It is very easy to confuse document control with change management. In terms of the world of medical devices the ISO13485 standard doesn’t really help. The text around change management is scant (34 words) and focuses more on the impact to the Quality Management System. There are over 370 words around how documents and records should be controlled. ISO14971, covering risk management, also focuses on the impact on the medical device and not how change is managed in an organisation per se.
Hardly sets a high expectation for making effective change that will last does it ?
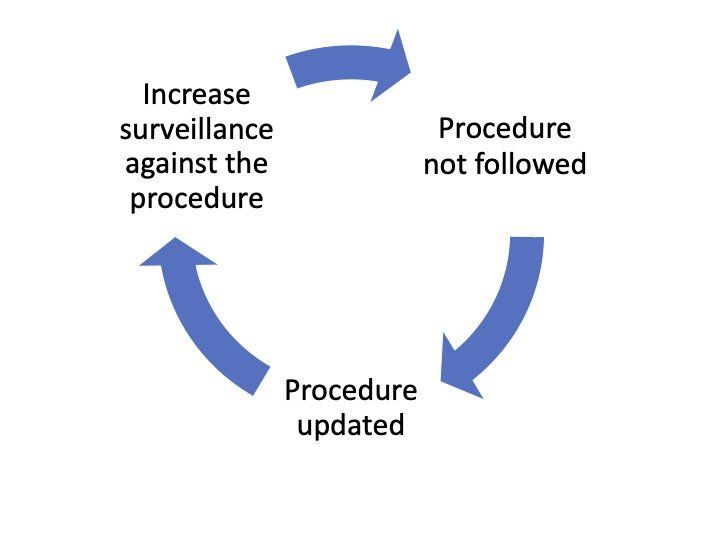
The consequence of this weakness is the documentation trap. Actions are buried within documentation. These actions are not followed. Procedures are further updated to fix this – the result of weak root cause investigation. Surveillance to check compliance is increased. Further deviations are observed and the cycle continues.
Organisations need to take change management seriously. The clue is in the title. Changing a document doesn’t change the world, it just changes the document.
So why do organisations confuse document control with change management ?
Well aside from the low bar set by the regulations, because it is hard.
The best change needs to involve the end-users as part of the change, ensure awareness of the need for change and impact to various stakeholders of not following the update. Training and assessment is needed to make sure that this is understood. It is far more painful than simply updating a piece of text in
Word.
You need to manage not only the risk of making that change, but the risk of making that change and it simply being ignored against the background of the enormous amount of information we have to navigate through every day.
But if effort is really put into change management, then you are more likely to get results you want.
The world of social policy has many examples where change happens not because of rules, but because change and expectations were proactively managed over time: speeding, passive smoking, plastic bag use..
Sadly, within the medical device world this documentation trap is happening at the macro level. Think MDR and IVDR. Rules and surveillance are being increased. Do you really think this will stop bad organisations and individuals criminally disregarding Good Manufacturing Practice and fraudulently manufacturing adulterated products ? I don’t think so.
So, the next time you want to implement a change ask yourself this: Do you want to change behaviours or just change a document ?
I know what I'd rather.